Lo Loestrin Fe is the only available ultra-low-dose oral contraceptive (OC) with just 10 mcg of daily estrogen1,2

*A 1-year (thirteen 28-day cycles), multicenter, open-label study of 1270 women aged 18-35 years (BMI ≤35 kg/m2) assessed efficacy of Lo Loestrin Fe for 12,482 twenty-eight-day
10 mcg is the lowest daily dose of ethinyl estradiol among all available OCs1,2
With 10 mcg of daily ethinyl estradiol in a 24/2/2 regimen, Lo Loestrin Fe has 38% less estrogen per month compared with a 20-mcg, 21/7 OC regimen1,7
Women may potentially take an OC for many years during their reproductive lives8
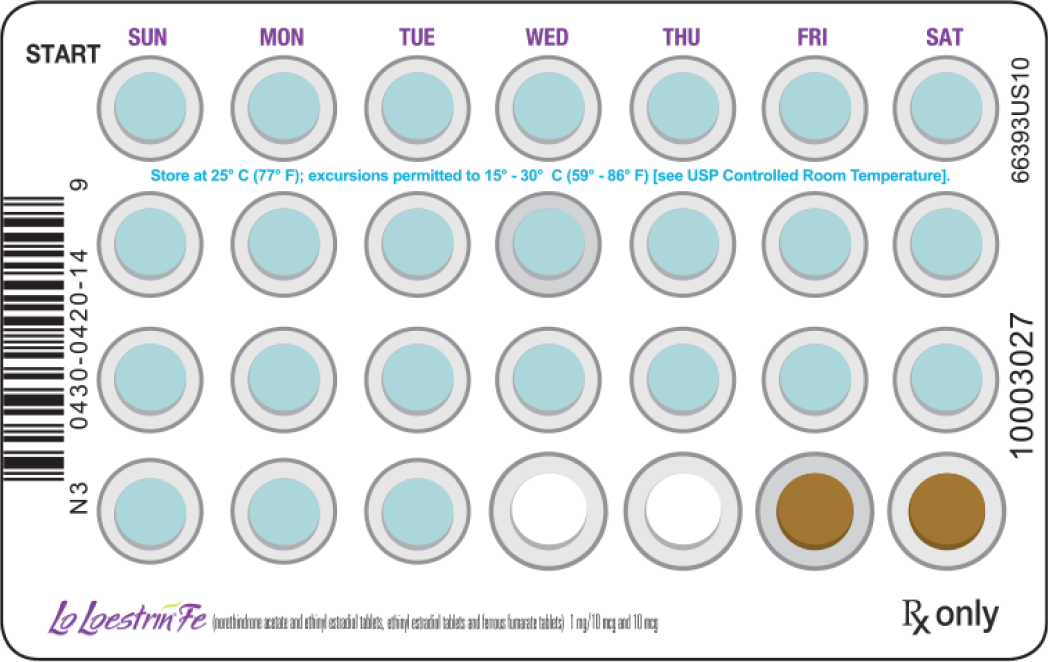
To achieve maximum contraceptive effectiveness, patients should be instructed to take:
- Lo Loestrin Fe exactly as directed
- One tablet by mouth at the same time every day
- Tablets in the order directed on the blister pack
Tablets should not be skipped or taken at intervals exceeding 24 hours. For patient instructions for missed pills, see FDA-approved Patient Labeling.
24 Blue Active Pills
1 mg norethindrone acetate and 10 mcg ethinyl estradiol
2 White Active Pills
10 mcg ethinyl estradiol
2 Brown Placebo Pills
75 mg ferrous fumarate
The ferrous fumarate tablets do not serve any therapeutic purpose.
Mean duration of withdrawal bleeding was <2 days per cycle1
Patient-reported intensity of withdrawal bleeding was lighter than normal5
Incidence of amenorrhea1,5,†
Incidence of amenorrhea increased over the course of the study, from 32% in Cycle 1 to 49% in Cycle 13
Breakthrough bleeding/spotting decreased over time1,5,‡§
- Mean number of intracyclic bleeding/spotting days decreased from 3.2 in Cycle 2 to 2.4 in Cycle 4, and 1.8 in Cycle 8
- 4% of women discontinued treatment due to bleeding irregularities

Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on combination oral contraceptives (COCs), especially during the first 3 months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. Counsel patients that amenorrhea may occur. Rule out pregnancy in the event of amenorrhea in 2 or more consecutive cycles.
†A subject was considered to have amenorrhea for any interval with no reported bleeding or spotting.
‡A 1-year (thirteen 28-day cycles), multicenter, open-label study of 1270 women aged 18 to 35 years designed to assess the efficacy of Lo Loestrin Fe for pregnancy prevention for a total of 12,482 twenty-eight-day evaluable cycles of exposure. Safety was evaluated in women aged 18 to 45 years.1
§Intracyclic bleeding was defined as any day in which a subject experienced bleeding of any intensity except light bleeding that did not require sanitary protection; such bleeding was defined as spotting. All bleeding (spotting) days and episodes were counted as intracyclic bleeding/spotting days unless it was the first bleeding episode starting after the last day of active drug intake and before the beginning of the next treatment cycle, or starting within 4 days of the last day of active drug intake and continuing at least through the first day after the end of active drug intake in the treatment cycle. Such bleeding was defined as withdrawal bleeding.5

Consider Lo Loestrin Fe for All Your Appropriate Patients
In the pivotal phase 3 clinical study, Lo Loestrin Fe was evaluated for pregnancy prevention, including:
- 1270 women aged 18 to 351
- BMI ≤35 kg/m2 (89-260 lbs)1,||
- Mean weight of 150 lbs.
- Patients who were recently postpartum (completed 2 or more normal menstrual cycles before enrollment)17
- Patients new to OCs (51%)¶
- Patients switching OCs (49%)17
1270 women
aged 18 to 351
BMI ≤35 kg/m2 (89-260 lbs)1,||
Mean weight of 150 lbs
Patients who were recently postpartum
(completed 2 or more normal menstrual cycles before enrollment)17
Patients new to
OCs (51%)¶
Patients switching
OCs (49%)17
||The efficacy of Lo Loestrin Fe in women with a BMI of >35 kg/m2 has not been evaluated. ¶New to OCs was defined as not using hormonal contraception at the time of study enrollment.17

